Durham-based 410 Medical, developer of a new medical device for treating critically ill patients, has raised $3.1 million in new financing to commercialize the product.
The funding round was led by the AIM Group, an angel investor network based in Opelika, Ala., that invests in life science and technology products in the Southeast. Other new investors include the North Carolina Venture Capital Multiplier Fund, Kleinheinz Capital and WakeMed Health & Hospitals, the health system where 410’s first product was developed.
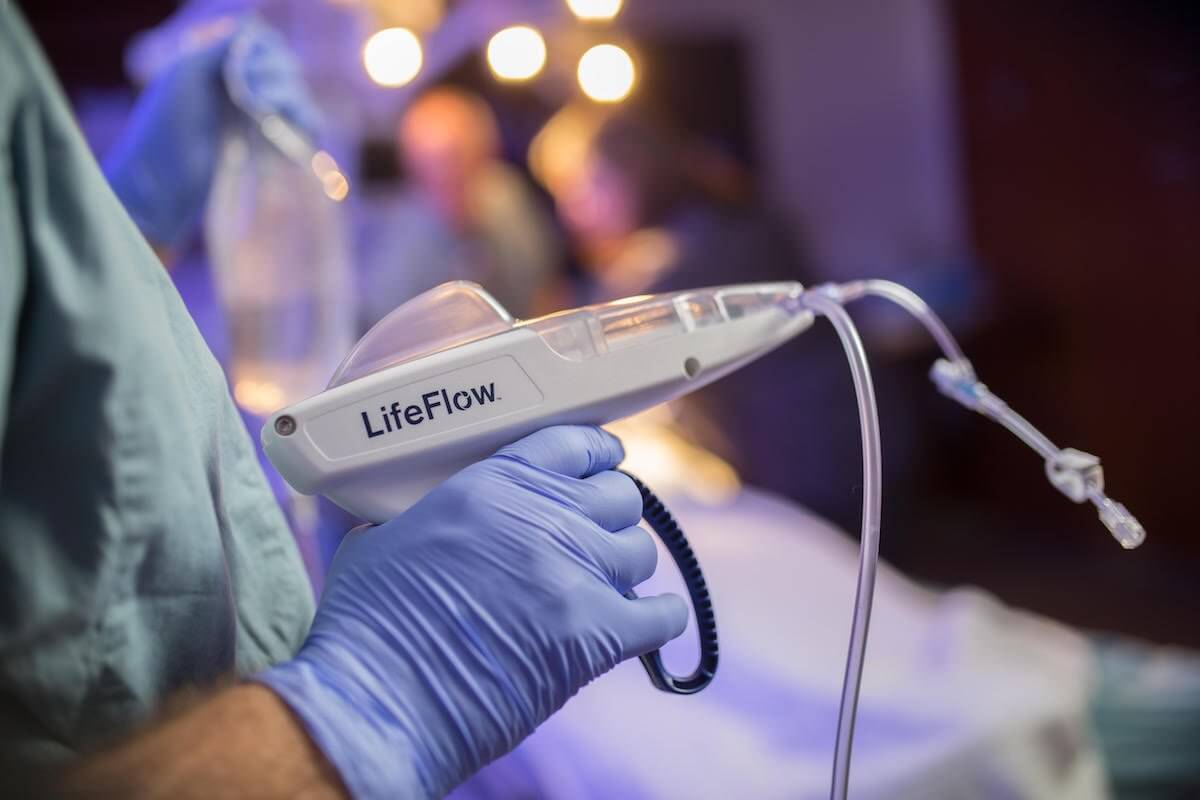
The product, LifeFlow, is a hand-operated device that allows health care providers to deliver fluids quickly and efficiently, improving care for patients with life-threatening conditions such as shock and sepsis. The product received clearance for human use in 2016 from the U.S. Food and Drug Administration.
“WakeMed’s investment is particularly exciting as it highlights the positive impact that LifeFlow is having for patients,” said Kyle Chenet, 410’s president and chief executive officer.
The company and the medical system have collaborated since the inception of LifeFlow by WakeMed physician Mark Piehl, who saw an opportunity to help critically ill patients including children with sepsis and other forms of shock.
“LifeFlow is a great example of a new product that is improving health and economic outcomes for our patients and our hospital,” said Denise Warren, WakeMed’s chief operating officer.
After FDA clearance and successful initial evaluation in 2017, WakeMed began using LifeFlow throughout its hospitals. The device is used frequently to treat patients with septic shock, trauma and other emergency conditions related to dangerously low blood pressure that can quickly lead to organ failure or death if not treated quickly.
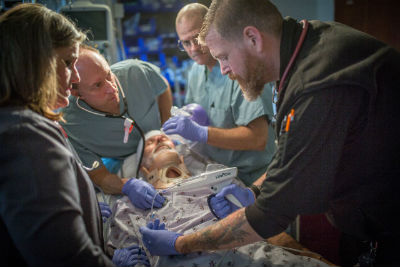
“Since the introduction of LifeFlow, nurses have been able to reduce the time required to deliver an initial dose of IV fluid by more than 50 percent,” said Jennifer Farmer, nurse manager in WakeMed Children’s Emergency Department. “We can now obtain IV access, complete the fluid bolus and administer antibiotics all within the ‘golden hour,’ which often leads to an immediate improvement in a patient’s condition.”
Farmer said that as familiarity with LifeFlow increases among providers, “it is becoming a standard for our sickest pediatric patients in the emergency department and is now even included in our pediatric crash carts.”
LifeFlow is faster, easier and more efficient than current methods of fluid delivery including gravity infusion, IV infusion pumps, pressure bags and manual syringes, 410 says on its website. The device can be readied for use in less than two minutes, can be operated by one hand and can deliver precise amounts of fluid with each squeeze of the handle.
Market expansion next
Upcoming milestones for LifeFlow’s continued development include completion of post-marketing clinical studies and continued growth in target markets, Chenet said. The device is being used or tested in more than 40 hospitals.

“Our mission is to get LifeFlow in the hands of the health care providers who are treating critically ill patients,” he said.
The device, intended for single patient use, is being manufactured in-state by Robling Medical of Youngsville.
In most cases LifeFlow is not eligible for separate payment or reimbursement by health insurers but is typically covered under a bundled payment structure, Chenet said.
An analysis of LifeFlow for the treatment of pediatric shock showed that use of the device can substantially lower expected hospital costs due to shorter patient stays and greater adherence to fluid-delivery guidelines.
410 Medical was established in 2013. In 2016 it was awarded a $250,000 Small Business Research Loan by the North Carolina Biotechnology Center, but the company subsequently decided to seek other funding. Since then, 410 has grown to include 15 employees.
The company was cofounded by Piehl and Luke Roush.
Piehl, who is 410’s chief medical officer, is a pediatric intensivist at WakeMed, where he was medical director of WakeMed Children’s Hospital from 2009 to 2015. He earned his medical degree and a master’s degree in public health from the University of North Carolina at Chapel Hill.
Roush is a managing principal and co-founder of the San Francisco venture capital firm Sovereign’s Capital, which has an operations office in Raleigh and is an investor in 410. A graduate of Duke University, Roush previously held positions at TransEnterix, a medical device company, and Liquidia Technologies, a drug-engineering company, both of Morrisville.





























